The Use of Biomarkers in Clinical Trials
Learning Objectives
- Sentence number one.
- Sentence number two.
- Sentence number 3.
The First Section
This document uses the markdown syntax. Read more about it here: https://help.github.com/articles/markdown-basics/.
Bold text can be created with double stars or double underscores.
Italic text can be created with single stars or single underscores.
Separate paragraphs with line breaks.
\(\alpha\)
Definition
Content
Some math: \(\int_0^1 \beta^2 f(x) \, df(x)\)
Aside or Note
content
Subsection
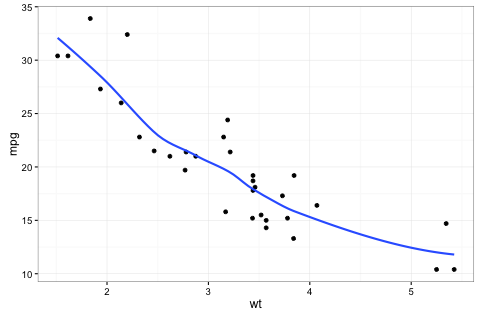
Example figure. Put the caption in between the square brackets.
Links can be added using pointy brackets http://www.google.com, or inline using the markdown syntax
Section 2
According to Jennings, Van Deerlin, and Gulley (2009). For references, use the “at” symbol, followed by the first authors last name, then the year, and finally the first non-article word of the title.
Test your knowledge
Click the question to reveal answer
References
Jennings, Lawrence, Vivianna M Van Deerlin, and Margaret L Gulley. 2009. “Recommended Principles and Practices for Validating Clinical Molecular Pathology Tests.” Archives of Pathology & Laboratory Medicine 133 (5): 743–55.
